|
|
Dardanus Lagopodes
Hairy Red Hermit Crab
|
|
Shaw Kwok 2015
|
|
|
|
Summary | |
Hermit crabs are recently known around 1400 species on the world and with different colours, pattern and choice of shells. They are mostly scavengers and detritivores, also able to change food preferences for new food source. Dardanus lagopodes belongs to the hermit crab family diogenidae. It is a red hairy hermit crab which belongs to a very diverse group called crustaceans. They usually live in inter-tidal, sand and rubbers to 100 meters sub-tidal area, and which are marine hermit crabs. However, D. lagopodes and other hermit crabs are not “true” crabs. The infraorder that given to hermit crabs is anomura, literally means “differently-tailed’. In fact, most of the hermit crabs are asymmetrical that the abdomen is unusual to other crustaceans, and having a soft and twisted abdomen. This is a big contrast to other family members of crustaceans, for example, shrimps and lobsters, when their abdomen are calcified and symmetrical. While comparing to “true” crabs, the abdomen of hermit crabs are elongated; the carapace are not fully calcified and unable to cover the whole body. Thus, hermit crabs have a very distinctive behavior that selecting gastropod shells for protection. Gastropod shells are crucial to hermit crabs, because they are not only provide shelter and protection, but also avoid desiccation and suffocation when they are moving at shallow-water habitat or even land. Especially, D. lagopodes inhabit a wide variety of habitats, including, sand and rubbles in intertidal zone, water carrying in their shells are very important. Yet, shell selection included some components that restricting the usage of shells by hermit crabs. Morphology and sex are the two major factors that affect shell choice. For instance, D. lagopodes are large size in diogenidae and flatten body plan which allows them to choose a wider range of shell species. Male hermit crabs grows bigger than females are also able to choose bigger shells species for fitness. The walking legs that designed for hermit crabs are best fit their living style in gastropod shells. Hermit crabs have only two pair of walking legs and two pair of reduced legs. The small legs are designed for adjusting the shell position when they are moving or protruding out the shells. For defense and offense, hermit crabs have one pair of claws to protect their life. However, hermit crabs need to moulting for body grow as similar as other animals of crustacean. This is the time that hermit crabs need to find a bigger shell and having no protection. In addition, hermit crabs are also vulnerable when they finished their larval stage to juvenile, because this is the first time that they pick their gastropod shells. The availability of gastropod shells are controlled by gastropod predators as hermit crabs cannot prey directly on them. Hermit crabs are attracted by odor from dead gastropod fresh and chemicals that released from shells to locate the shells. Therefore, gastropod predation sites are often aggregated with large amount of hermit crabs. Fighting for shells and changing shells are the two mainly activities for marine hermit crabs and since million years ago. Recently, hermit crabs are being debated with the “carcinization” hypothesis, which some people believe that they are the ancestor of king crabs. In this study, there is a little project about the carcinization and to investigate whether D. lagopodes relate to king crabs.
|
|
|
Physical Description | |
In general, diogenidae is the family which contains most of the large size intertidal hermit crabs (Poore 2007). They are mostly left-handed (left chela is bigger) or having both claws with similar size; the left claw in some species are not significantly larger than the right claw, for example, D. lagopodes (Jones & Morgan 2002). In contrast with other family members, paguridae are right-handed hermit crabs and are smaller in body size than the hermit crabs belong to diogenidae (Jones & Morgan 2002).
D. lagopodes are very hairy, and with reddish brown body colour. Therefore, D. lagopodes is called Hairy Red Hermit Crab. The hair is useful on detecting physical contact with external factors. An adult can grow to body size around 8 centimeters long, which is larger among other genus of hermit crabs (Jones & Morgan 2002). For this specimen, the body size is around 6 centimeters which is assumed an adult form (figure 3). The size of left claw is larger than the right claw but not significant. There are 5 pairs of walking legs and one pair of degenerated legs which is grown at the ventral side between abdomen and cephalothorax (Figure 1). The soft abdomen is twisted to the right (figure 1 & 2) and smooth which do not contain pleopods. The absence of pleopods indicate that this is a male where mostly female in some species have pleopods that grow at the side of abdomen (Fantucci, Biagi & Mantelatto 2009) (figure 2). Anus is at the tip of the abdomen.
Carapace is reddish brown and white with a purplish-red patch anteriorly. The stalk of eye is yellow colour at dorsal view and white colour at ventral view. Antennular and antennal are yellow with olive green lines. Pereopods including claws are mottled red, brown and white. Anterior dorsal shield of carapace is purple and blue; posterior carapace is mottled red, brown and white. The abdomen is deep purple and reddish brown; the tip of abdomen is olive green with white telson and uropod.
Compound eye: distinguishing distance within 20 – 30 meters and colours on gastropoda’s shell with yellow, blue and grey
Antannae: a physical sensory organ to detect movement and vibration
Antennule: a chemosensory organ that use for distance chemoreception
Maxilliped: to draw food particles into the mouth
Pereopod 1 (Claws): use to defence and for offensive purpose
Pereopod 2 & 3: the longest pairs of legs for walking
Pereopod 4 & 5: a reduced pairs of legs which use to assist movement with the shell
Gonophore: Male-intersex gonophore (non-functional)
Anterior carapace: an anterior shield to cover the cephalothorax
Posterior carapace: a posterior shield to cover the back of cephalothorax
Pleopods: only females contain pleopods that use for carrying eggs
Uropods: A tail fan that grow on the left and right side of telson for locking up the position in shells
Telson: The tip of exoskeleton that covers anus
(Source Tudge 1995, Ruppert, Fox & Barnes 2004)



|
 |
| Figure 1 |
|
 |
| Figure 2 |
|
 |
| Figure 3 |
|
|
|
Ecology | |
Interaction with gastropods

The ecology of hermit crabs are closely related to gastropod shells that are available in the marine or terrestrial environment. Shells are very important as they are vulnerable with soft and uncalcified abdomen. Without shells predators are easily prey on them. Interestingly, hermit crabs are able to find their new shells after moulting and growing. They need to changing shells that correspondingly to enlarged body size. Different species of hermit crabs have vary morphological design which influence the decision on choosing the best fit of shells. Commonly, D. lagopodes lives in broad-mouthed shells, such as syrinx, tectus, latirus, angaria and lambis species (Tudge 1995). In addition, the distinct flattening carapace of D. lagopodes allow them to occupy narrow-mouthed shells either (figure 4).
As mentioned in the physical description, hermit crabs can see the color on gastropods shell within 20 – 30 meters. However, hermit crabs do not simply rely on their compound eyes for choosing the best fit of shells. Chemical cues (odor) allows hermit crab to locating empty shells. The empty shells of gastropod is able to release some chemical signals to hermit crabs by different sources, for example, the emanation of calcium ions from the surface of the shells or partly digested snail fresh or dead body (Tricarico, Breithaupt & Gherardi 2011). Gastropod predation sites can lead to a huge aggregation of hermit crabs. Once they pick the favorite shell, immediately escape from the site (Tricarico & Gherardi 2006). Hermit crabs are unable to prey directly on snail for a new shell as they are not sensitive to the odor of living gastropods (Tricarico, Breithaupt & Gherardi 2011). Thus, the predators of gastropoda control the availability of shells to hermit crabs. A good quality of shell is depend on the cause of death of gastropods. Hermit crabs will not pick a destructed shells or shells with holes for their new house, and this is control by the vary species of predators (Turra, Denadai & Leite 2005).
Interaction with conspecifics
Shell exchanges are less likely to happen between hermit crabs (Tricarico & Gherardi 2006). In the reason of quality and the best fitness of shells for individuals are so rarely available in the natural environment. Actually, hermit crabs are generalist to many resources but unambiguously not including food and empty shells (Barnes 2003). The competition and shell-fighting behavior are usually happen in the life cycle of hermit crabs, because they need to fight for the best fit of shells which related to their own shape, size and weight (Childress 1972). However, terrestrial hermit crabs are more preferred used shells from conspecifics because each occupants will remodel their shells for better protection (Laidre & Trinh 2014). As marine hermit crabs do not prefer used shells and choices are limited by shell availability, they have to be expand their choice with their living habitat (Tricarico & Gherardi 2006). The hermit crabs which live in heterogeneous habitat are discovered to be more diverse on picking the shell species, and in contrast with the homogeneous living hermit crabs (Teoh, Hussein & Chong 2014). Therefore, if a hermit crab can live with a variety of habitat, it has stronger ability to survive and avoid competition with intra or inter species. This explain why D. lagopodes can occupy a large variety of shells with their large size and flatten shape.
Feeding style
Most of the intertidal hermit crabs can feed on mangrove detritus, phytoplankton and benthic microalgae, specifically benthic microalgae contribute the most energy consumption of hermit crabs (Teoh & Chong 2015). However, hermit crabs can change their feeding preferences with availability of food. Marine hermit crabs are omnivorous filter feeders but also an active detritivores and scavengers in intertidal environment for dead organisms (Laidre & Greggor 2015). This can also explain why hermit crabs can react so quickly to empty shells that left by gastropod, as the odor of carrion is so attractive to them. Interestingly, hermit crabs have the ability to find new food sources as the stress of finding food sources in the environment increased. Hermit crabs are more likely to digest novel food than many other scavengers, and they can based on the result of their physiological state to value the preferences of food (Tran 2015).
Predators
Predators of hermit crabs are mostly fishes, lobster, octopus and crabs. In general, there are two type of predators that prey on hermit crabs. First type of predator is shell-crushing type predators, for example, calappids, xanthids, spiny lobsters and fish (Brooks & Mariscal 1985). Second type of predator is non-shell-crushing type predators, for example, octopuses can pull the hermit crabs out from the shells by using the sectorial arms (Brooks & Mariscal 1985).
|
 |
| Figure 4 |
|
|
|
Life History and Behaviour | |
Larval development
Unfortunately, there is absent of embryonic developmental research about the D. lagopodes. However, the embryonic development of D. lagopodes can be studied by looking at other family and genus members as they are sharing the same ancestor. In general, there are 7 stages before zoea hatching from eggs (figure 5).
Stage I Zygote and cell division (1st cleavage)
Stage II Blastula and follow with gastrulation
Stage III Forming yolk-free area (germinal disc)
Stage IV Increasing the yolk-free area from 5% to around 60%
Stage V Initiation of eyes and heart
Stage VI Increasing the yolk-free area to around 90% and eyes are clearly observed
Stage VII Clearly see the shape of zoea and ready to hatch
Table 1. The 7 stages of embryonic development of tropical intertidal hermit crab (Decapoda, Anomura) (Turra & Pereira Leite 2007; Roberts, Eisenhour & I’Anson 2014)

Temperature has an effect on the larval development of hermit crabs. As there are sub-tropical, tropical, marine and semi-terrestrial hermit crab species, temperature is a crucial element on controlling the biodiversity of larva. Some experiments have already taken on tropical hermit crabs with the same family level (Diogenidae) as D. lagopodes. The temperature graph (Figure 7) shows a significant difference of development rate at different temperature from 12 – 30 degree Celsius and the development rate of all stages increase with the increasing of temperature.
Breeding of hermit crab start when the ovigerous female carrying eggs on their pleopods, and the breeding season of each species of hermit crab are different (Reese 1968). Awkwardly, no research on the breeding season about the D. lagopodes. However, the breeding period of hermit crabs are continuously throughout the year, but each species have different peak and trough of breeding rate (Reese 1968). The two species of diogenid hermit crab Diogenes brevirostris and D. deformis share the same peak time of breeding period around August to October (Harms 1992; Litulo & Tudge 2005). D. lagopodes may have a peak breeding time around the same period as they (D. brevirostris and D. deformi) are at same genus level. The productive activity is different between species, even intra-population and they are triggered by different degree of temperature and external factors, for example rainfall (Harms 1992).
Behaviour
Anti-predator behaviour
Gastropod shells are crucial to hermit crabs, therefore they will compete for the fitness or optimal shells which suit for their specific needs, for example, distinct body size, shape and avoiding predators. Moreover, some intertidal hermit crabs rely the shell for respiration when they move on mud and mangroves, without shells they can be desiccated and suffocated (Rotjan, Blum & Lewis 2004; Turra & Gorman 2014; Alcaraz, Chavez-Solis & Kruesi 2015). Surprisingly, some research discovered that some species of hermit crabs would abandon their shells if they were facing some level of threats and stimuli. Inter tidal hermit crabs Pagurus criniticornis abandon their shells rapidly (no matter optimal or sub-optimal shells) when receives suddenly and potentially fatal risk, for example, sand burial or trapping (Turra & Gorman 2014). In contrast, some individuals will keep hiding in their shells when receives lower degree of threats, for example, entrapment (Turra & Gorman 2014). This behavior shows that hermit crabs are able to justify the level of threats to their life and decide whether to abandon shells. According to Kuhlmann (1992) mentioned that some hermit crabs prefer sand burying more than picking optimal shells for anti-predator response, for example, P. longicarpus which lives in sandy intertidal zone. Hermit crabs may have different anti-predator behavior which associated with the habitat and hunting strategies of predators, optimal shells selection is not the only defense strategies of hermit crabs (Bertness 1981b). D. lagopodes may also equipped with the sand burying behavior as they are also living in intertidal habitat and diversity of habitat. Unfortunately, there are no research on this species and further experiment is needed to test the anti-predator of D. lagopodes.
Shell selection behavior
Shell selection is a type of natural selection that hermit crabs use different type of gastropod shells to protect their body, soft abdomen and eggs from predators, and avoid environmental disturbance (Lively 1988). Hermit crabs have the ability to select gastropod shells with vary of shape, size, colour, mass and species (Reese 1962; Reese 1963; Lively 1988). Turra & Leite (2004) concluded that the selection of shell species are based on the body size and weight of hermit crabs. In addition, Alcaraz, Chavez-Solis & Kruesi (2015) discovered that aquatic hermit crabs prefer heavy shells in contrast with semi-terrestrial prefer light shells. In the reason of aquatic hermit crabs have higher frequency of predation rather than competition, they needs higher mass of shells for protection more than rapid growth of body size for competition (Alcaraz, Chavez-Solis & Kruesi 2015). Furthermore, shell sizes and shapes influence the body growth and morphology of hermit crabs (Turra & Leite 2003; 2004). The selection of shells lead to a consideration for hermit crab whether shell species provide the greatest fitness. Male hermit crabs are able to carry larger and heavier shells than females as they grow larger in size and having longer intermolt periods (Turra & Leite 2003). The male D. lagopodes in this study has also equipped with a large shells which is heavy and robust. The video below shows the cone shell that carried by D. lagopodes.
<table style="margin: 0px auto;">
<tbody>
<tr>
<td>
<iframe width="560" height="315" align="centre" frameborder="0" src="//www.youtube.com/embed/0BgjeRiJ7ts"></iframe>
</td>
</tr>
<tr>
<td align="center"><span style="font-size: 11px;">
Video 1. Dardanus lagopodes with heavy cone shell
</td>
</tr>
</tbody>
</table>
|
 |
| Figure 5 |
|
 |
| Figure 6 |
|
 |
| Figure 7 |
|
|
|
Anatomy and Physiology | |
Circulatory system
Hermit crabs and other crustacean species are having the same circulatory system which is an open or lacunar type circulatory system. Open circulatory system is different to the closed circulatory system as mammals. The system has no veins and no separation of fluid (blood) from interstitial fluid. Hemolymph is the fluid (blood) that go through the whole body of hermit crabs (figure 8). In the open circulatory system, a heart is presented for pumping the hemolymp throughout the artery to hemocoel then return to the heart with sinus. There are a lot of small sinus connected to the gills which responsible for oxygen and carbon dioxide exchange and transferring oxygenated blood back to heart by pericardial sinus. (Roberts, Eisenhour & I’Anson 2014)
Nervous system
The most important nervous system of hermit crab is the compound eye and antennae. In the reason of they rely on these two sensory features to locate and define the fitness of shells. Specially, the antennae is fully responsible the sense of odor which released from dead gastropods and predators, even the minerals on shells (figure 9) (Groh et al. 2014).
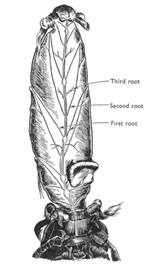
The abdominal nervous system pair with the abdominal muscle to allowing different degrees of turning of abdomen. The abdomen of hermit crabs is very important on support the shells height and weight. The abdominal muscle needs to work with the 3rd and 4th peropods in regulating shell posture and height away from substrate (Chapple & Krans 2004). The central nervous system is connect with three roots of alternative fibers that connecting ventrally and posterior of abdomen (figure 10). In addition, the abdominal nervous system also responsible for the pleopods of females that carrying eggs. The abdomen is lacked of sensory hair which is a big contrast to the anterior of body. Instead of sensory hairs, epidermal receptors are the dominant sensors where the abdomen is decalcified in contrast with the calcified peropods and claws (Chapple 1966).
Digestive and excretory system
The digestive system of hermit crabs contain foregut, midgut and hindgut (figure 8). Hermit crabs use the third hairy maxillipedes to scrap the food particles and transfer particles to the mouth like a brush by using the hair (Orton 1927). Also hermit crabs can sifting and selecting food particles through rejection which rejected particles will borne forwards and upwards in water (Orton 1927). The stomach is located under the heart where the foregut posterior chamber is. The injected food particles will pass to the midgut stomach to the anus through hindgut intestine (figure 8 & 11).
Respiratory system
The gills of hermit crabs are similar to the members of anomura which is a phyllobranchiate gills (figure 12). The water will draw into the gill cavity at the branchial chamber by scaphognatite (a thin leaflike appendage) and abdominal movements for air breathing (Freire, Onken & McNamara 2008). Aquatic hermit crabs have lower hemolymph (blood) flow than land or terrestrial crabs for reducing respiratory evaporative water loss as air has higher oxygen content than water (Hsia et al. 2013).

The sexual maturity and sexual dimorphism are hardly to determine because hermit crabs are intersexual. The intersexual means an individual contain both female and male reproductive organs. Hermaphroditism associated with the intersexuality in hermit crabs because they can switch sex in the different periods of lifetime (Sagi et al. 1996; Turra 2007). Hermit crabs are certain with non-functional hermaphroditism which containing male intersexes (figure 13) and female intersexes (figure 14): an individual can contain both sexual reproductive system but one of them is not functional (Turra 2004; Turra 2005). Pleopods are functional as egg carrying features but males are also having pleopods in some species for driving water motion in the shell for cleaning and water circulation (Morgan 1989). However, the process of reproduction of hermit crabs are profoundly studied. Basically, spermatophores are male sex organ with ampulla that for sperm transfer on female sex organ (gonophore). The spermatophore is formed by a protective and adhesive materials that surround the spermatozoa (Subramanian, 1991). During the ejaculation, males will attach the spermatphores on to the gonophore and produce a mass of sticky materials which stored with sperm (Hess & Bauer, 2002).

Then the eggs will release by the female for joining the spermatphores mass, and spawning occur within a few hours after fertilization of eggs (Amadio & Mantelatto 2009). The spermatophore of D. lagopodes (figure 15) is very distinctive to other paguroidea which having two ampullae on a very long stalk (peduncle) (Amadio & Mantelatto 2009). The spermatophore is about 600 micrometers long from the base (pedestal) with 70 micrometers long ampullae. However, the D. lagopodes in this study does not develop with pleopods but it owns a gonophore. The male gonophore may not be a functional sex organ. For the species that be able to perform functional intersexuality, the oogenesis occurring in the testes (Sant’Anna, Turra & Zara 2010). The (figure 14) show the gonophore is connected to testis through vas deferens.
|
 |
| Figure 8 |
|
 |
| Figure 9 |
|
 |
| Figure 10 |
|
 |
| Figure 11 |
|
 |
| Figure 12 |
|
 |
| Figure 13 |
|
 |
| Figure 14 |
|
 |
| Figure 15 |
|
|
|
Evolution and Systematics | |
Taxonomic hierarchy – D. lagopodes
Phylum: Arthropoda
Involving insects, crabs, lobsters, shrimp, spiders, mites, horseshoe crabs and they have: Most of them are having jointed appendages, tagmata, body form (head, thorax, abdomen or cephalothorax and abdomen), cuticular exoskeleton, and open circulatory system. (Roberts, Eisenhour & I’Anson 2014)
Subphylum: Crustacea (10 classes)
Most of them are having two pairs of antennae, one pair of mandibles, two pairs of maxillae, body segment around 16 – 20 and each segment contain a pair of appendages, head and body fused (cephalothorax) and larva is Nauplius. (Roberts, Eisenhour & I’Anson 2014)
Class: Malacostraca (16 orders including shrimps, lobsters, crabs & krill etc)
Most of them are having 8 segments on thorax, 6 or 7 segments on abdomen with telson and uropod, nonrespiratory and respiratory functional carapace, carapace is bivalved and enveloping most of the body or only covering the throax or short and covering only the anterior most of the thoracic segments. (Roberts, Eisenhour & I’Anson 2014)
Order: Decapoda (krills, lobsters, shrimps and crabs)
Deca(ten)poda(legs) is coming from the 5 pairs of appendages (pereiopods or peropods), 3 pairs of mouthparts (maxillipeds), 1 pair of claws (chalae), abdomen contain biramous pleopods, telson with uropods (Gary & Nikos 2014)
Infraorder: Anomura (7 superfamily includes hermit crabs, porcelain crabs and mole crabs)
Crabs like form but not true crabs, the first two pairs of peropods are much larger than the rest, the abdomen can be large and elongated (hermit crabs) or small and hide under the thorax (king crabs). (Provenzano & Rice 1966)
Superfamily: Paguroidea
All hermit crabs are belonged to this superfamily with elongated soft uncalcified abdomen which protected under gastropod shells, sponge, tube-worm tube or hollow rock, 4 pairs of legs on cephalothorax: 2 pairs of walking legs and the rest 2 pair of appendages are responsible for controlling the shells position, pleopods absence or presence on both sex in some species, usually present on the left side of abdomen of female for carrying eggs (Provenzano & Rice 1966).
Family: Diogenidae
The left claw (chalae) is larger than the right, body with brightly colour, some species are large in body size. (Provenzano & Rice 1966; Jones & Morgan 2002; Poore 2007)
Genus: Dardanus spp.
Chalae usually covered with spines and hair, left chalae is larger than or similar to right, carapace length is the longest in diogenidae and with large body size. (Jones & Morgan 2002)
Species: Dardanus lagopodes
Left and right chalae are similar size, body are deep red-brown colour with long reddish hairs. (Jones & Morgan 2002)
Little project – Carcinization “Hermit crabs to King crabs”
The phylogeny of hermit crabs are well studied, and they are belongs to the anomura which is the monophyletic group to brachyura (true crabs) and they lay within the decapoda. Inside the group of anomura, there are seven superfamily groups. Paguroidea (hermit crabs) and lithodoidea (king crabs) are sister group and they contain and share a common ancestor of all members in the group (figure 16). Specifically, some research believe that lithodoidea is evolved from the paguroidea based on the morphological and molecular levels.
Carcinization is the biological term that referring some lobster-like crustacean towards a crab-like formation (Borradaile, 1916). In the 19th century, Borradaile (1916) observed that most of the animals with broadened carapace, flattened body shape and reduced pleon or abdomen may evolve from an ancestor with elongated body plan, for example, shrimps, lobster, crayfish and hermit crabs (figure 17). The common shallow water hermit crab Pagurus longicarpus has prompted the evolutionary dynamics of hermit crabs as intrapopulation show the shell-loss and reduction of shell use (Blackstone 1985;1989).This concept has approved and believed by many biology scientists based on comparing the difference of animals on morphological and molecular level in decapoda (Cunninghame, Blackstone & Buss 1992; McLaughlin, Lemaiture & Sorhannus 2007; Remimann et al. 2011; Tsang, Chan, Ahyong & Chu 2011; Bracken-Grissom et al. 2013; Keiler, Richter & Wirkner 2013). Especially, hermit crabs and king crabs are suggested to be closely related and king crabs are possibly evolved from hermit crabs.
King crabs are among the largest crustaceans and have more crab-like body plan. In contrast with the hermit crabs which is smaller in size and relying an empty shell for protection. Basically, the carcinization has involved some steps before becoming a more crab like form. First of all, the carapace must be short and flat; secondly, the carapace must be wide enough to cover the whole body form and with folded pleon (Scholtz 2014). Numerous studies are agreed that pagurids (family) of hermit crabs nested with the lithodids (king crabs).
However, the carcinization of hermit crabs was seriously debated that whether hermit crabs evolve to king crabs (“hermit to king hypothesis”) or king crabs evolve to hermit crabs (“king to hermit hypothesis”) (Bracken-Grissom et al. 2013). Despite, there are lacking of fossil records and living animals with partial carcinized body plan, but fossil records of hermit crabs appear earlier than king crabs (Scholtz 2014; Tsang, Chan, Ahyong & Chu 2011; Bracken-Grissom 2013). The fossil record support the pagurus were emerged at the early Jurassic and followed with the lithodids emerged from the Miocene (figure 18) (Tsang, Chan, Ahyong & Chu 2011; Bracken-Grissom 2013). Fossil record and DNA sequences is also a robust evidence to support the carcinization theory. Cunninghame, Blackstone & Buss (1992) shows that molecular evidence (DNA sequence) indicated the king crabs are nested with pagurus hermit crab by encoding the mitochondrial large-subunit ribosomal RNA which shows a high correlation between geological time and the DNA sequences. Hemolymph vascular system is another focus on studying the carcinization for paguridae. The both taxa shows correspondences in the morphology of the arterial systems and adjacent anatomical structures (Keiler, Richter & Wirkner 2013). Unfortunately, diogenidae did not show a close relationship with lithodoidea and further investigation is needed.

|
 |
| Figure 16 |
|
 |
| Figure 17 |
|
 |
| Figure 18 |
|
|
|
Biogeographic Distribution | |
The geographical distribution of D. lagopodes (figure 19) are mainly tropical where can find at Australia, Japan, Madagascar, Malaysis, Malaysia, Philippines, Red Sea, Republic of Mauritius, Samoa, Seychelles, South Pacific Ocean, Taiwan, East Africa and Indo-Pacific (World Registor of Marine Species 2015) .

In Australia, D. lagopodes dominated the Queensland (figure 20) intertidal zone and Great Barrier Reef, some of them can find at North and Western Australia (The Atlas of Living Australia 2015; Jones & Morgan 2002).

|
 |
| Figure 19 |
|
 |
| Figure 20 |
|
|
|
Conservation and Threats | |
Global warming
Shell selection behavior is a distinctive behavior of hermit crabs which is significantly different to other animals in crustacean. Gastropod shells are very important to hermit crabs as they rely on it for protection, respiration (land hermit crabs) and avoid desiccation. Global warming is threatening numerous of species around the world in recent years, even accelerate the warming of temperature and reduction of pH value in sea water. The reduction of pH in sea water is a threat to hermit crabs. According to de la Haye et al. (2011) stated that the reduction of sea water pH can influence the shell choice of Pagurus bernhardus hermit crabs that the hermit crab lives in lower pH sea water cannot normally switch the sub-optimal shell to optimal shells. Thus, the reduction of sea water pH can disrupts the decision-making and shell selection behavior of hermit crabs.
Chemical contamination
The common antifouling substance tributyltin (TBT) and butyltin (BT) are often used to prevent sessile organisms grow on boat hulls (Godoi, Favoreto & Santiago-Silva2003a). However, these substances can give ovary disorganization and atrophy in hermit crabs (Sant’Anna, Santos & Marchi 2014). Heavy metal is found to be highly toxic to hermit crabs Clibanarious africanus, which are zinc sulfate (ZnSO4) and followed with copper sulfate (CuSO4) and cadmium sulfate (CdSO4) (Otitoloju & Don-Pedro 2006).
Conservation
To dueling with the threats to hermit crabs, global warming and chemical contamination are the first two issues. For global warming, the reduction of carbon dioxide emission is crucial on improving the reduction of pH in sea water. For chemical contamination, the antifouling substance should be inhibited and replaced with environmental friendly materials. The heavy metal that release to the estuaries or ocean must first be monitored and controlled with laws and policy.
|
|
|
References | |
- Alcaraz, G, Chávez-Solís, CE & Kruesi, K 2015, ‘Mismatch between body growth and shell preference in hermit crabs is explained by protection from predators’, Hydrobiologia, vol. 743, no. 1, pp. 151-156.
- Amadio, LM. & Mantelatto, FL 2009, ‘Description of the Male Reproductive System of the Hermit Crab Calcinus tibicen (Decapoda: anomura: diogenidae)’, Journal of Crustacean Biology, vol. 29, no. 4, pp. 466-475.
- Anger, K 1989, ‘Growth and exuvial loss during larval and early juvenile development of the hermit crab Pagurus bernhardus reared in the laboratory’, Marine Biology, vol. 103, no. 4, pp. 503-511.
- Anger, K, Montú, M & de Bakker, C 1990, ‘Energy partitioning during larval development of the hermit crab Pagurus bernhardus reared in the laboratory’, Journal of experimental marine biology and ecology, vol. 141, no. 2, pp. 119-129.
- Barnes, DKA 2003, ‘Local, regional and global patterns of resource use in ecology: hermit crabs and gastropod shells as an example’, Marine Ecology Progress Series, vol. 246, no. 1, pp.211-223.
- Bartilotti, C, Calado, R & dos Santos, A 2008, ‘Complete larval development of the hermit crabs Clibanarius aequabilis and Clibanarius erythropus (Decapoda: Anomura: Diogenidae), under laboratory conditions, with a revision of the larval features of genus Clibanarius’, Helgoland Marine Research, vol. 62, no. 2, pp. 103-121.
- Bertness, MD 1981b, ‘Predation, physical stress, and the organization of a tropical rocky intertidal hermit crab community’, Journal of Ecology, vol. 62, no. 1, pp. 411-425.
- BlackstoneE, NW 1985, Hermit crabs and carcinization: trends in the development and evolution of some Decapod Crustaceans (Pagurus, Long Island sound), ProQuest, UMI Dissertations Publishing, United States.
- Blackstone, NW 1989, ‘Size, shell-living and carcinization in geographic populations of a hermit crab, Paguvus hivsutiusculus’, Journal of Zoology, vol. 217, no. 1, pp.477-490.
- Borradaile, LA 1916, ‘Porcellanopagurus: an instance of carcinization’, Zoology, vol. 3, no. 1, pp. 111-126.
- Bracken-Grissom, HD, Cannon, ME, Cabezas, P, Feldmann, RM, Schweitzer, CE, Ahyong, ST, Felder, DL, Lemaitre, R & Crandall, KA 2013, ‘A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda)’, BMC evolutionary biology, vol. 13, no. 1, pp. 128-128.
- Brooks, WR & Mariscal RN 1985,‘Protection of the hermit crab Pagurus Pollicaris say from predators by hydroid-colonized shells’, Journal of experimental marine biology and ecology, vol. 87, no. 2, pp. 111-118.
- Chapple, WD 1966, ‘Sensory modalities and receptive fields in the abdominal nervous system of the hermit crab, Pagurus granosimanus (Stimpson)’, The Journal of experimental biology, vol. 44, no. 2, pp. 209.
- Chapple, WD & Krans, JL 2004, ;Cuticular receptor activation of postural motoneurons in the abdomen of the hermit crab, Pagurus pollicarus’, Journal of Comparative Physiology A, vol. 190, no. 5, pp. 365-377.
- Childress, JR 1972, ‘Behavioral Ecology and Fitness Theory in a Tropical Hermit Crab’, Ecology, vol. 52, no. 5, pp. 960-964.
- Cunningham, CW, Blackstone, NW & Buss, LW 1992, ‘Evolution of king crabs from hermit crab ancestors’, Nature, vol. 355, no. 6360, pp. 539-542.
- de la Haye, KL, Spicer, JI, Widdicombe, S & Briffa, M 2011, ‘Reduced sea water pH disrupts resource assessment and decision making in the hermit crab Pagurus bernhardus’, Animal Behaviour, vol. 82, no. 3, pp. 495-501.
- Fantucci, MZ, Biagi, R & Mantelatto, FL.2009, ‘Use of pleopod morphology to determine sexual dimorphism and maturity in hermit crabs: Isocheles sawayai as a model’, Helgoland Marine Research, vol. 63, no. 2, pp. 169-175.
- Freire, CA, Onken, H & McNamara, JC 2008, ‘A structure–function analysis of ion transport in crustacean gills and excretory organs’, Comparative Biochemistry and Physiology, Part A, vol. 151, no. 3, pp. 272-304.
- Godoi, AFL, Favoreto, R & Santiago-Silva, M 2003a, ‘Environmental contamination for organotin compounds’, Quimica Nova, vol. 26, pp.708-716.
- Groh, KC, Vogel, H, Stensmyr, MC, Grosse-Wilde, E & Hansson, BS 2014, ‘The hermit crab's nose—antennal transcriptomics’, Frontiers in Neuroscience, vol. 7, no.1, pp. 1-12.
- Harms, J 1992, ‘Larval development and delayed metamorphosis in the hermit crab Clibanarius erythropus (Latreille) (Crustacea, Diogenidae)’, Journal of experimental marine biology and ecology, vol. 156, no. 2, pp. 151-160.
- Hess, GS & Bauer RT 2002, ‘Spermatophore transfer in the hermit crab Clibanarius vittatus (Crustacea, Anomura, Diogenidae)’, Journal of Morphology, vol. 253, no. 1, pp. 166-175.
- Hsia, CCW, Schmitz, AS, Lambertz, M, Perry, SF & Maina, JN 2013, ‘Evolution of Air Breathing: Oxygen Homeostasis and the Transitions from Water to Land and Sky’, Comprehensive Physiology, vol. 3, no. 2, pp. 849-915.
- Jones, DA & Morgan, GJ, A field guide to crustaceans of Australian waters, Western Australian museum, Western Australia.
- Keiler, J, Richter, S & Wirkner, CS 2013, ‘Evolutionary morphology of the hemolymph vascular system in hermit and king crabs (Crustacea: Decapoda: Anomala)’, Journal of Morphology, vol. 274, no. 7, pp. 759-778.
- Kornienko, ES & Korn, OM 2011, ‘The larval development of the hermit crab Areopaguristes nigroapiculus (Decapoda: Anomura: Diogenidae) reared under laboratory conditions’, Journal of the Marine Biological Association of the United Kingdom, vol. 91, no. 5, pp. 1031-1039.
- Krieger, J, Sombke, A, Seefluth, F, Kenning, M, Hansson, BS & Harzsch, S 2012, ‘Comparative brain architecture of the European shore crab Carcinus maenas (Brachyura) and the common hermit crab Pagurus bernhardus (Anomura) with notes on other marine hermit crabs’, Cell and tissue research, vol. 348, no. 1, pp. 47-69.
- Kuhlmann, ML 1992, ‘Behavioral avoidance of predation in an intertidal hermit crab’, Journal of experimental marine biology and ecology, vol. 157, no. 2, pp. 143-158.
- Litulo, C. & Tudge, C. 2005, ‘Population structure and breeding season of the hermit crab Diogenes brevirostris Stimpson, 1858 (Decapoda, Anomura, Diogenidae) from southern Mozambique’, Journal of Natural History, vol. 39, no. 31, pp. 2887.
- Lively, CM 1988, ‘A Graphical Model for Shell-Species Selection By Hermit Crabs’, Ecology, vol. 69, no. 4, pp. 1233-1238.
- McLaughlin, PA, Lemaitre, R & Sorhannus, U 2007, ‘Hermit crab phylogeny: A reappraisal and its “Fall-Out”’, Journal of Crustacean Biology, vol. 27, no. 1, pp. 97-115.
- Morgan, GJ 1989, ‘The hermit crabs (Decapoda: Anomura; Diogenidae, Pahuridae) of southwestern Australia, with descriptions of two new species’, Records of Western Australia Museum, vol. 14, no. 3, pp. 391-417.
- Onken, H & Riestenpatt, S 1998, ‘NaCl absorption across split gill lamellae of hyperregulating crabs, transport mechanisms and their regulation’, Composition of Biochemistry and Physiology A, vol. 119, no. 1, pp.883-893
- Orton, JH 1927, "On the Mode of Feeding of the Hermit-crab, Eupagurus Bernhardus, and some other Decapoda", Journal of the Marine Biological Association of the United Kingdom, vol. 14, no. 4, pp. 909-921.
- Otitoloju, AA & Don-Pedro, KN 2006, ‘Determination of types of interactions exhibited by binary mixtures of heavy metals tested against the hermit crab, Clibanarius africanus’, Toxicological & Environmental Chemistry, vol. 88, no. 2, pp. 331-343.
- Poore, GCB 2007, Crabs, hermit crabs and allies, Museum Victoria, Melbourne.
- Poupin J 2012, Crustacea database, Viewed 16 March 2015, <http://decapoda.free.fr/illustration.php?n=6&sp=224>
- Provenzano, AJ & Rice, AL 1966, ‘Juvenile Morphology and the Development of Taxonomic Characters in Paguristes sericeus A. Milne-Edwards (Decapoda, Diogenidae)’, Crustaceana, vol. 10, no. 1, pp. 53-69
- Reese, ES 1968, ‘Annual breeding seasons of three sympatric species of tropical intertidal hermit crabs, with a discussion of factors controlling breeding’, Journal of experimental marine biology and ecology, vol. 2, no. 3, pp. 308-318.
- Reimann A, Richter S, Scholtz G. 2011, ‘Phylogeny of the Anomala (Crustacea, Decapoda, Reptantia) based on the ossicles of the foregut’, Zoologischer Anzeiger, vol. 250, no. 1, pp. 316-342.
- Richter & Scholtz 2001, ‘Phylogenetic analysis of the Malacostraca (Crustacea)’, Journal of Zoological Systematics and Evolutionary Research, vol. 39, no. 3, pp. 113-136.
- Roberts, H, Eisenhour, K & I’Anson, L 2014, ‘Intergrated Principles of Zoology’, 16th edn, McGraw-Hill Education, New York.
- Rotjan, RD, Blum, J & Lewis, SM 2004, ‘Shell Choice in Pagurus longicarpus Hermit Crabs: Does Predation Threat Influence Shell Selection Behavior?’, Behavioral Ecology and Sociobiology, vol. 56, no. 2, pp. 171-176.
- Reese, ES 1962, ‘Shell selection behaviour of hermit crabs’, Animal Behaviour, vol. 10, no. 3, pp. 347-360.
- Reese, ES 1963, ‘The Behavioral Mechanisms Underlying Shell Selection by Hermit Crabs’, Behaviour, vol. 21, no. 1/2, pp. 78-126.
- Ruppert, EE, Fox, RS, & Barnes, RD 2004, ‘Invertebrate Zoology: A Functional Evolutionary Approach’, 7th edn, Brooks/Cole, Belmont, United States.
- Sagi, A, Khalaila I, Barki A, Hulata, G & Karplus, I 1996, ‘Intersex red claw crayfish, Cherax quadricarinatus (Von Martens): functional males with pre-vitellogenic ovaries’, Journal of Biology, vol. 190, no.1, pp. 16-23.
- Sant’Anna, BS, Santos, DM, Marchi, MRR, Zara, FJ & Turra, A 2014, ‘Surface-sediment and hermit-crab contamination by butyltins in southeastern Atlantic estuaries after ban of TBT-based antifouling paints’, Environmental Science and Pollution Research, vol. 21, no. 10, pp. 6516-6524.
- Sant’Anna, B, Turra, A & Zara, F 2010, "Simultaneous activity of male and female gonads in intersex hermit crabs", Aquatic Biology, vol. 10, no. 3, pp. 201-209.
- Scholtz, G. 2014, ‘Evolution of crabs – history and deconstruction of a prime example of convergence’, Contribution of Zoology, vol. 83, no. 2, pp. 87-105.
- Siddiqui, FA, McLaughlin, PA & Crain, JA. 1993, ‘Larval development of the hermit crab Clibanarius albidigitus (Crustacea: Anomura: Diogenidae) reared under laboratory conditions’, Marine Biology, vol. 116, no. 4, pp. 603-613.
- Subramoniam, T 1991, Chemical composition of spermatophores in decapod crustaceans, pp. 587-608, Columbia University Press, New York.
- Teoh, HW & Chong, VC 2015, ‘Versatile hermit crabs harness multiple-source energy from coastal mudflats: implications for fish production’, Aquatic Ecology, vol. 49, no. 1, pp.43-55.
- Teoh, HW, Hussein, MAS & Chong, VC 2014, ‘Influence of habitat heterogeneity on the assemblages and shell use of hermit crabs (Anomura: Diogenidae)’, Zoological Studies, vol. 53, no. 1, pp. 1-9.
- The Atlas of Living Australia 2015, Viewed 29 May 2015, <http://spatial.ala.org.au/?q=lsid:%22urn:lsid:biodiversity.org.au:afd.taxon:afacab16-9953-42ea-8765-bdf22b8947b0%22&cm=geospatial_kosher>
- Tran, MV 2015, ‘Behavioral reactions to novel food odors by intertidal hermit crabs’, Behavioural Processes, vol. 113, no. 1, pp. 35-40.
- Tricarico, E, Breithaupt, T & Gherardi, F 2011, ‘Interpreting odours in hermit crabs: A comparative study’, Estuarine, Coastal and Shelf Science, vol. 91, no. 2, pp. 211-215.
- Tricarico, E & Gherardi, F 2006, ‘Shell Acquisition by Hermit Crabs: Which Tactic Is More Efficient?’, Behavioral Ecology and Sociobiology, vol. 60, no. 4, pp. 492-500.
- Tsang, LM, Chan, T, Ahyong, ST & Chu, KH 2011, ‘Hermit to King, or Hermit to All: Multiple Transitions to Crab-like Forms from Hermit Crab Ancestors’, Systematic Biology, vol. 60, no. 5, pp. 616-629.
- Turra, A 2004, ‘Intersexuality in hermit crabs: reproductive role and fate of gonopores in intersex individuals’, Journal of marine biology, vol. 84, no.1, pp. 757-759.
- Turra, A 2005, ‘Reproductive behavior of intertidal hermit crabs in south-eastern Brazil’, Journal of marine biology, vol. 22 no.1, pp. 313-319.
- Turra, A, Denadai, M & Leite, F 2005, ‘Predation on gastropods by shell-breaking crabs: effects on shell availability to hermit crabs’, Marine Ecology Progress Series, vol. 286, no. 1, pp. 279-291.
- Turra, A & Gorman, D 2014, ‘Subjective resource value and shell abandoning behavior in hermit crabs’, Journal of experimental marine biology and ecology, vol. 452, pp. 137-142.
- Turra, A & Leite, F 2003, ‘The molding hypothesis: linking shell use with hermit crab growth, morphology, and shell-species selection’, Marine Ecology Progress Series, vol. 265, pp. 155-163.
- Turra, A & Leite, FPP 2004, ‘Shell-size selection by intertidal sympatric hermit crabs’, Marine Biology, vol. 145, no. 2, pp. 251-257.
- Turra, A & Leite, FPP 2007, ‘Embryonic development and duration of incubation period of tropical intertidal hermit crabs (Decapoda, Anomura)’, Revista Brasileira de Zoologia, vol. 24, no. 3, pp. 677-686.
- Tudge, CC 1991, ‘Spermatophore Diversity within and among the Hermit Crab Families, Coenobitidae, Diogenidae, and Paguridae (Paguroidea, Anomura, Decapoda)’, Biological Bulletin, vol. 181, no. 2, pp. 238-247.
- Tudge, CC 1995, Hermit crabs of the Great Barrier Reef and coastal Queensland, School of Marine Science, The University of Queensland, Brisbane.
- Laidre, ME & Greggor, AL 2015, ‘Swarms of swift scavengers: ecological role of marine intertidal hermit crabs in California’, Marine Biology, vol. 162, no. 5, pp. 969-977.
- Laidre, ME & Trinh, R 2014, ‘Unlike terrestrial hermit crabs, marine hermit crabs do not prefer shells previously used by conspecifics’, Crustaceana, vol. 87, no. 7, pp.856-865.
- World Register of Marine Species 2015, Viewed 29 May 2015, <http://spatial.ala.org.au/?q=lsid:%22urn:lsid:biodiversity.org.au:afd.taxon:afacab16-9953-42ea-8765-bdf22b8947b0%22&cm=geospatial_kosher>
|
|
|
|
|